Desmoid tümörlerde yeni tedavi seçeneği olarak nirogacestat
Desmoid tümörlerde yeni tedavi seçeneği olarak nirogacestat
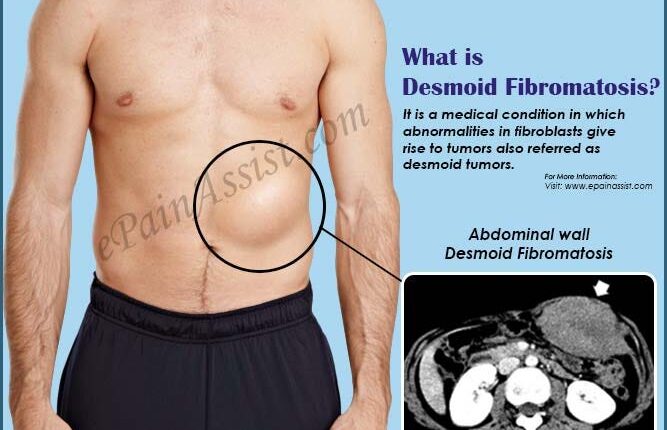
Desmoid tümörler ya da agresif fibromatozis, lokal seyreden, yavaş büyüyen bağ dokusu tümörleridir.
Desmoid tümörler, metastaz yapmaz fakat ortaya çıktığı organın fonksiyonunu bozan ciddi problem yaratan tümörlerdir
Desmoid tümörlerde NOTCH reseptör yolağının belirgin artığı biliniyordu. Notch yolağı inhibisyonu bu tümörler için önemli bir strateji olduğu biliniyordu.
Nirogacestat adlı molekül γ-secretase inhibisyonunu yapan günde 150 mg iki kez alınan bir tirozin kinaz inhibitörü. İlaç, Notch yolağı aktivasyonunda önemli rol alan γ-secretase inhibe ederek tümör progresyonu engellediği ve gerilettiği saptandı.
Nirogacestat adlı molekül, daha önce tedavi almış ve hastalık nüksü göstermiş hastalarda hastalıksız süreyi önemli oranda artırdığı ve yeni olay oluşumunu %80 oranında azalttığı saptandı.
İlaç ile ilişkili yönetilebilir yan etkiler olarak, ishal, mukozit, karın ağrısı gibi semptomlar saptandı.
Nirogacestat adlı ilacın, desmoid tümörlerde etkili olduğunu gösteren DeFi çalışması sonrası, Amerika Birleşik Devletleri Gıda ve İlaç Dairesi (FDA) tarafından kullanılması onaylandı.
Kaynak
By The ASCO Post Staff
FDA Approves Nirogacestat for Desmoid Tumors
On November 27, the U.S. Food and Drug Administration (FDA) approved the selective gamma secretase inhibitor nirogacestat (Ogsiveo) for adult patients with progressing desmoid tumors who require systemic treatment. This is the first approved treatment for desmoid tumors.
DeFi Trial
Efficacy was evaluated in DeFi (ClinicalTrials.gov identifier NCT03785964), an international, multicenter, 1:1 randomized, double-blind, placebo-controlled trial in 142 patients with progressing desmoid tumors not amenable to surgery. Patients were eligible if the desmoid tumor had progressed within 12 months of screening. Patients were randomly assigned to receive 150 mg of nirogacestat or placebo orally twice daily until disease progression or unacceptable toxicity.
The major efficacy outcome measure was progression-free survival based on Response Evaluation Criteria in Solid Tumors version 1.1 as assessed by blinded independent central review or on clinical progression by the investigator (and adjudicated by independent review). Median progression-free survival was not reached in the nirogacestat arm (95% confidence interval [CI] = not reached to not reached) and 15.1 months (95% CI = 8.4 months to not reached) in the placebo arm (hazard ratio [HR] = 0.29, 95% CI = 0.15–0.55, P < .001). An exploratory analysis of progression-free survival based on only radiographic progression demonstrated a hazard ratio of 0.31 (95% CI = 0.16–0.62).
Objective response rate was an additional efficacy outcome measure. Objective response rate was 41% (95% CI = 29.8%–53.8%) in the nirogacestat arm and 8% (95% CI = 3.1%–17.3%) for those receiving placebo (P < .001). Additionally, efficacy results were supported by change from baseline in patient-reported worst pain favoring the nirogacestat arm.
The most common adverse reactions in patients receiving nirogacestat were diarrhea, ovarian toxicity, rash, nausea, fatigue, stomatitis, headache, abdominal pain, cough, alopecia, upper respiratory tract infection, and dyspnea.
The recommended nirogacestat dose is 150 mg administered orally twice daily with or without food until disease progression or unacceptable toxicity. Each 150 mg dose consists of three 50 mg tablets.
This review used the Real-Time Oncology Review pilot program, which streamlined data submission prior to the filing of the entire clinical application, and the Assessment Aid, a voluntary submission from the applicant to facilitate the FDA’s assessment.