Hormon pozitif meme kanseri ve Küçük hücreli dışı akciğer kanserinde yeni bir tedavi seçeneği olarak Datopotamab deruxtecan
Hormon pozitif meme kanseri ve Küçük hücreli dışı akciğer kanserinde yeni bir tedavi seçeneği olarak Datopotamab deruxtecan
Kanser tedavisinde son yıllardaki en önemli gelişmelerden biri, kanser hücrelerinde yoğun bulunan proteinlere karşı geliştirilmiş antikor-sitotoksik tedavi kombinasyonlarıdır.
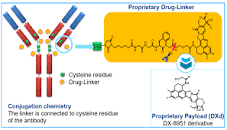
Antikor-sitotoksik kombinasyonu, kanser hücrelerinin yüzeyindeki hedeflenen proteine bağlanır, sitotoksik kemoterapi kısmı endoliz ile kanser hücresine girer ve hücre içinde aktive olarak kanser hücresinin ölümüne neden olur.
Bu yöntemle normal hücreler korunmuş, kanser hücreleri hedeflenerek yok edilmiş olur. Diğer önemli sonucu, bir tümör hücresini öldüren sitotoksik kemoterapi yan taraftaki kanser hücresine sızarak etkisini devam etmesi ile beklenen etkinlik normal kemoterapiye göre çok artmaktadır.
Datopotamab deruxtecan, meme ve akciğer kanserinde bol sentezlenen TROP2 proteine karşı geliştirilmiş antikor yapısı ve kanser hücrelerinin gelişimi için önemli olan topoizomeraz 1 enzimini inhibe eden sitotoksik kemoterapi kompleksinden oluşur.
Son yapılan çalışmalarda, tedavi seçeneği tükenmiş hormon pozitif meme kanseri hastalarında Datopotamab deruxtecan sık kullanılan kemoterapilere karşı daha etkili olduğu ve hastalıksız süreyi uzattığı gösterilmiştir.
Akciğer yapılan çalışmada, tedavi seçenekleri tükenmiş hastalarda Datopotamab deruxtecan, dosetaksel kemoterapisine göre daha iyi yanıt ve hastalıksız süreyi uzattığı görüldü. Bu etkinliği skuamöz hücreli olmayan grupta daha belirgin olduğu saptanmıştır.
Kaynaklar
Clinical trial identification
NCT05104866
Clinical trial identification
NCT04656652.